Navigating FTO in the Pharmaceutical Industry: Challenges and Strategies

The pharmaceutical sector stands at the intersection of science and business, balancing patient impact with profit incentives. Companies invest billions discovering and developing new drugs – but realizing returns means navigating an intricate web of intellectual property (IP) rights.
Enter freedom to operate (FTO) searches. By comprehensively investigating patents and regulatory exclusivities, FTO provides the information pharma leaders need to chart strategic courses to market.
This guide explores the unique complexities of FTO in pharma, equipping you with actionable frameworks to direct patent strategy. Let’s examine key challenges, best practices, and real-world case studies to master IP navigation.
Introduction: The Pivotal Role of Patents in Pharma
Patents fuel pharma. These time-limited IP monopolies allow companies to reclaim massive R&D investments by preventing copying. In exchange, inventors must fully disclose technical details that enable future innovation.
Over 290,000 pharma patents are filed annually in the US alone. Applications begin early, even before human trials, to stake claims. Approval establishes exclusivity, though disputes frequently arise.
Navigating this landscape is treacherous but essential. Just ask Pfizer, which braved a decade-long patent battle over blockbuster drug Lyrica, ultimately saving billions in revenue.
FTO searches are crucial for analyzing patents, litigation risks, and market exclusivities. By directing R&D priorities and deal-making, high-quality FTO offers a strategic advantage.
The Challenges of Pharmaceutical FTO

Patent Protection Permutations
Pharma products seemingly sprout infinite patents. Discrete aspects – compounds, delivery mechanisms, production processes – become claims in an interlocking fortress.
FTO investigations must probe countless permutations. Related molecules. Novel salts, polymorphs, isomers, and formulations expand protection. So do patented analytic techniques, equipment configs, and precision delivery mechanisms.
Complicating matters, worldwide regimes establish jurisdiction-specific patents, notwithstanding international filings. Each represents potential dispute flashpoints requiring verification.
Pharmaceutical FTO regularly tracks hundreds of interrelated patent families relevant to a drug. Focused human analysis filters signals from noise.
Of course, patents aren’t pharmaceuticals’ only protections.
The Regulatory Labyrinth
Data exclusivities imposed by the FDA provide separate, additive monopolies preventing generic drug approvals. FTO searches must account for these, too.
Key exclusivities include:
- 5-Year New Chemical Entity: Shielding new compounds. Bars submissions rely on innovator safety/efficacy data.
- 3-Year New Clinical Study: Protecting supplemental indications and formulations. Using original clinical data is also prohibited.
- 7-Year Orphan Drug: Incentivizing rare disease R&D. Blocks rivals using original data.
- 12-Year Biologic: Safeguarding innovator’s license applications. Biosimilars must be referenced afterward.
Smart FTO analysis melds patent intelligence with regulatory timelines for fully informed projections. Missing vital exclusivities leaves money on the table.
Global Strategy Development
Pharma’s worldwide reach introduces geographic nuances galore. Regulatory exclusivities and patent laws diverge internationally, demanding localized FTO.
While the US currently dominates biopharma innovation, companies still target approvals across triad markets of North America, Europe, and Japan to maximize returns.
Harmonizing global IP takes finesse. Patents might be issued in some areas but not others. Approval lags between countries also alter market entry timings.
Plus, healthcare systems wield varying influence over pricing and generics penetration, adjusting commercial outlooks.
FTO searches must incorporate these moving parts for multi-dimensional insights. Regional variances could make or break competitive positions.
With challenges in focus, let’s pivot to solutions.
Strategic Approach to Pharmaceutical FTO
Comprehensive Searches
Combing global patent portfolios touching proposed therapeutics requires an organized methodology:
Landscape Framing – Surface-level queries of emerging art in disease areas, mechanisms, and modalities. Highlights crowds.
Freedom Assessments – Targeted probes on exact molecules/methods of interest. Seeks direct claims to priority patents.
Portfolio Reviews – Inspecting applicant citations and related families. Traces threads to originators.
Invalidation Searching – Scrutinizing art potentially invalidating identified patents. Builds a defensive arsenal.
Regulatory Analytics – Mapping relevant data and market exclusivities tied to assets. Notes gaps.
Litigation Monitoring – Tracking lawsuits involving priority patents and parties. Warns of disputes.
Synchronizing Protection Analysis
Regulatory and IP protections cannot be evaluated in isolation. Their codified interplay allows comprehensive planning:
- Clinical data exclusivities often outlast relevant patents, plugging gaps. But generics can carve out unconquered areas like new formulations.
- Patent term extensions may push boundaries beyond the base 20 years. But they don't override regulatory market exclusivities, already delaying submissions.
- Orphan drug exclusivity blocks other clinical studies using original data for seven years. It does not limit patent challenges to compound claims.
With so many levers pulling market access in opposing directions, integrated analysis is essential for accurately projecting openings. Carefully choreographing FTO procedures maximizes returns through orchestrated evergreening.
Navigating Patent Thickets & Evergreening
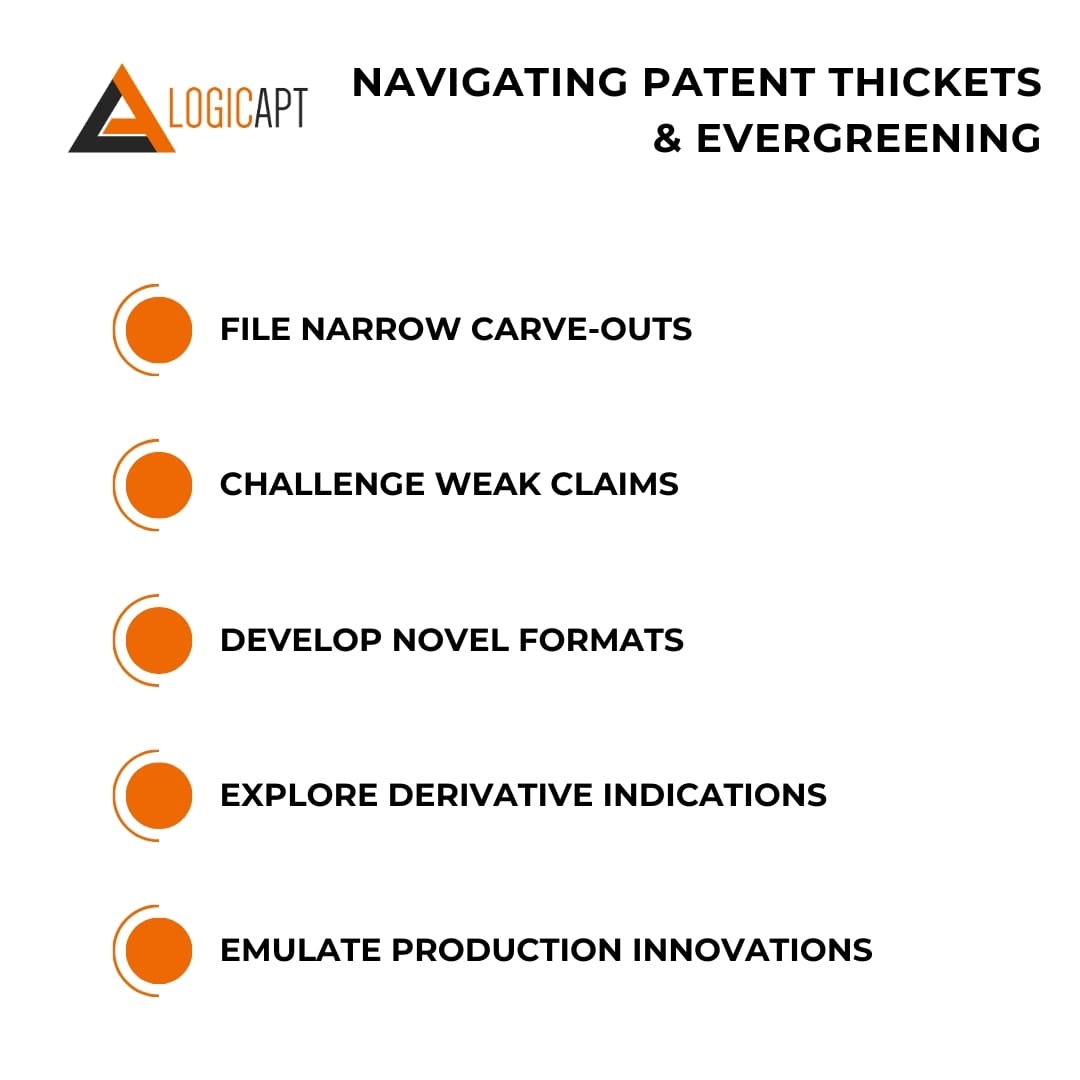
With so much profit at stake, coming up against elaborate blockade strategies in FTO searches is virtually guaranteed. Companies liberally patent incremental advances in drug delivery mechanisms, precision formulations, convenient combinations, and upgraded production processes specifically to obstruct.
But patent thickets can often be navigated by surveying boundaries. Even the densest clusters contain openings for clever maneuvering if you follow the content and extend lifecycles playbook:
- File narrow carve-outs – Stake out defensible positions outside overgrown areas through sharply defined applications.
- Challenge weak claims – Target vulnerable breadth lacking inventiveness for invalidations, clearing ground.
- Develop novel formats – Circumvent formulation and device patents via new presentations.
- Explore derivative indications – Repurpose for separate medical uses without disease treatment protections.
- Emulate production innovations – Design creative workarounds or joint process optimizations when possible.
Escapes can be spotted with attentive FTO guiding observation of maze edges instead of random entry points.
FTO In Action – Real-World Pharma Cases
Let’s examine events in the trenches illuminating effective FTO and early-stage patenting:

The ROS1 Inhibitor Runaround
When Pfizer sought to expand its lung cancer blockbuster Xalkori into ROS1-positive tumors in 2016, its FTO search uncovered an extensive patent estate from Shire Pharmaceuticals around key selective ROS1 inhibitors.
Rather than directly challenging this intellectual barricade, Pfizer pivoted to file applications on distinct soluble crystalline forms outside described molecules. Though initially denied on obviousness grounds, savvy, valid modifications yielded vital workarounds for production.
The refined tactics cleared space for Xalkori to claim new indication territory worth billions. But absent meticulous FTO guiding the pivots, revenues may have stalled.
Gilead’s Clinic flank on Sovaldi
When Gilead’s hepatitis C cure Sovaldi neared approval in 2013, AbbVie had method-of-treatment patents set to endure through 2028 around combination therapies with key antiviral components.
By scouring FTO details, though, Gilead spotted clinic-based loopholes allowing administration as solo therapies while still delivering high efficacy. Quick footwork filing a new regimen secured earlier market access.
Though ultimately blocked from first-line therapies for years, the creative FTO flanking still helped Sovaldi grab a substantial share through off-label administration and compassionate use—first-sighted freedom searching again paid dividends.
Best Practices & Future Outlook
Tips for Airtight FTO Execution
- Invest early & update often – Prioritize FTO during preclinical stages when more degrees of freedom exist. But continue revalidating with new art.
- Maximize global input – Seek guidance from jurisdictional experts worldwide to avoid contradictory regional strategies.
- Model complex scenarios – Simulate various launch timings, term extensions, invalidations, etc. to stress test plans.
- Automate monitoring – Use AI to continuously scan new patents, deals, lawsuits, etc., for alerts to reassess openings.
- Architect ironclad applications – Design original high-quality filings, constructing creative barriers against future competitors when claiming space.
The Future of FTO
Exponential expansions in biopharmaceutical complexity and personalization will demand even more surgical FTO. Key dimensions to watch:
- Biologics Upsurge – As the focus shifts from small molecules to biologics, thicker data exclusivity shields will ascend, requiring coordinated patent navigations.
- Personalized Pairings – Combination therapies for genetically defined patient subgroups will multiply FTO intricacies with interdependent diagnostic and therapeutic considerations in play.
- AI-Assistance – Automated analytics leveraging neural networks and machine learning will grow in tracing relationships within snarled patent networks and forecasting optimization pathways.
Conclusion – The Quest for Freedom
This deep dive has hopefully hammered home the indispensability of FTO for pharmaceutical feats. As scientific capabilities widen, so too will intellectual property thickets enclosing innovations.
With billions at stake commercializing new life-saving and life-enhancing therapies, the need for expert patent navigation will only intensify. Companies able to master strategic freedom searching will continue reaping outsized rewards.
But FTO is no longer just a back-end legal exercise. Astute firms now recognize assessing opportunities early and often provide a competitive advantage that ripples the entire enterprise.
The quest for freedom demands advanced preparation. But for those litigation-wary pioneers willing to chart courses minding margins, rich rewards await in making scientific imagination a reality.